Vaccinations Training
Training for Pharmacists for the Supply and Administration of Vaccinations

Pharmacists who have completed the required training, are permitted to supply and administer the following vaccines:
- seasonal influenza vaccine,
- pneumococcal polysaccharide vaccine
- herpes zoster (shingles) vaccine
- COVID-19 vaccines: used in the COVID-19 Vaccination Programme
(Updated December 2024):
- Comirnaty JN.1 3mcg (for children aged 6 months to 4 years)
- Comirnaty JN.1 10mcg (for children aged 5-11 years)
- Comirnaty JN.1 30mcg (for individuals 12 years and older)
- Comirnaty KP.2 30mcg (for individuals 12 years and older)
- Nuvaxovid XBB.1.5 (for individuals 12 years and older)
The legislation which allows for these services is available on the Irish Statute Book website.
Comprehensive information for pharmacists on the training required to supply and administer certain vaccines and/or emergency medicines is set out below. You may also find useful information in our FAQs section below which are updated regularly in response to queries we receive.
FAQs on COVID-19
In order to supply and administer any COVID-19 vaccine, pharmacists must first undertake or have up-to-date training in the following programmes:
- CPR for adults and children
- Responding to an Emergency Situation and Management of Anaphylaxis (RESMA)
- Medicines Administration (Parenteral) (PAMT)
- Delivery of a Pharmacy-Based Vaccination Service
- Vaccine-specific training developed by the National Immunisation Office (NIO) for the COVID-19 vaccine* to be administered (accessed on HSeLanD)
*All pharmacists administering COVID-19 vaccines should review the relevant associated HSeLanD module.
Please refer to the training diagram and table below which sets out which HSeLanD module you need to complete for each COVID-19 vaccine available.
Information on the training programmes approved by PSI, as well as the providers and how to book these courses is available on the Irish Institute of Pharmacy (IIOP) website. The IIOP has also developed a new vaccination training identification tool to guide and support pharmacists in identifying their training requirements for the supply and administration of vaccinations.
To access the NIO training programmes on HSeLanD, go to the Courses tab > Catalogue > Clinical Skills > COVID-19 Vaccination Training Programme. As well as completing the relevant training detailed above, pharmacists must also complete a self-assessment of competence.
The Medicinal Products (Prescription and Control of Supply) (Amendment) (No.7) Regulations 2020 (S.I. No. 698 of 2020) and subsequent amendments allow a registered pharmacist to supply and administer a COVID-19 vaccine where they have received training approved by the PSI. Further details of the PSI approved training for pharmacists is available here.
In order to supply and administer any COVID-19 vaccine, pharmacists must first undertake or have up-to-date training in the following programmes:
- CPR for adults and children
- Responding to an Emergency Situation and Management of Anaphylaxis (RESMA)
- Medicines Administration (Parenteral) (PAMT)
- Delivery of a Pharmacy-Based Vaccination Service
- Vaccine-specific training developed by the National Immunisation Office (NIO) for the COVID-19 vaccine to be administered (accessed on HSeLanD)
Information on the training programmes approved by PSI, as well as the providers and how to book these courses is available on the Irish Institute of Pharmacy (IIOP) website.
The National Immunisation Office (NIO) training programmes for the specific COVID-19 vaccines you wish to administer are available through HSeLanD- the Health Service Executive's online learning and development portal. The NIO training module you are required to undertake, depends on the COVID-19 vaccine product(s) you will be administering.
The HSE have developed this guide for community pharmacists to access HSeLanD training.
No. The Medicines Administration (Parenteral)(PAMT) training programme is not valid indefinitely. The PSI’s guidance on this is as follows:
Pharmacists are asked to reflect, self-assess and to evaluate whether they need to refresh their training in this programme, in order to ensure they have the necessary skills and knowledge to safely deliver the associated medicines or vaccination service.
The PSI requires that this training programme is repeated if a pharmacist wishes to administer:
- a vaccine or emergency medicine via an injection route i.e., intramuscular or subcutaneous, which they have never previously administered.
- or a vaccine or emergency medicine via an injection route which they have neither practised (i.e., administered to a patient) nor been trained in the previous 12 months (or in the case of seasonal influenza vaccination, in the previous flu season).
Please note that ‘trained in’ refers to training in the Medicines Administration (Parenteral) Training Programme. Therefore, there are times where the training may need to be repeated throughout the pharmacist’s career.
The Medicines Administration (Parenteral) (PAMT) course, which is accredited by the PSI, includes training on administration of injections to infants from 6 months of age. When offering a vaccination service for children, pharmacists should carefully reflect on their skills, and self-assess and evaluate whether they need to refresh their training with this programme. Pharmacists who have not completed the PAMT training programme, which commenced in 2016, must undertake this training programme in order to be able to administer vaccinations by injection to children from 6 months of age.
No. In order to supply and administer any vaccine, pharmacists must first undertake or have up-to-date training in the following programmes:
- CPR for adults and children
- Responding to an Emergency Situation and Management of Anaphylaxis (RESMA)
- Medicines Administration (Parenteral) (PAMT)
- Delivery of a Pharmacy-Based Vaccination Service
The training programmes for the specific vaccines you wish to administer can then be completed.
Information on the training programmes approved by PSI, as well as the providers and how to book these courses is available on the Irish Institute of Pharmacy (IIOP) website.
Further information on training for pharmacists on the supply and administration of vaccinations, including the validity period of each training programme is available on the PSI website.
Yes. COVID-19 vaccines (and influenza vaccines, see below) can be administered by pharmacists at any suitable and appropriate place, having regard to public convenience and the need to protect the health and safety of the public and safely administer the product.
We have published guidance to help you in considering what types of location are appropriate, and to support pharmacies in providing safe vaccination services offsite from the pharmacy premises. There are a number of checklists included in the guidance and key points for consideration. Current PSI guidance which supports the provision of a pharmacy vaccination service in the pharmacy will still apply and must be read and adapted to the chosen setting.
In order to be eligible to become a vaccinator employed by the HSE, a pharmacist must be up to date in CPR training for adults and children and Responding to an Emergency Situation and Management of Anaphylaxis (RESMA), Medicines Administration (Parenteral) (PAMT) and Delivery of a Pharmacy-Based Vaccination Service training programmes. You can check the period of validity of these training programmes on this website.
The relevant NIO COVID-19 vaccine training programme on HSeLanD must also be completed. All pharmacist vaccinators employed by the HSE should complete a self-assessment of competence, attesting to the completion of their training and competence. The HSE will also have particular governance arrangements and additional training requirements set out for those employed as vaccinators.
Yes. If you have never vaccinated before, or if you have not vaccinated in the last 12 months/during the last influenza season, you must complete the requisite training and complete a self-assessment of competence.
What training has been approved by the PSI?
The PSI has approved training in respect of a significant number of COVID-19 vaccine products since the commencement of the national COVID-19 Vaccination Programme. The list of COVID-19 vaccine products currently being used in the national COVID-19 Vaccination Programme, for which PSI has approved training, is available on the PSI website.
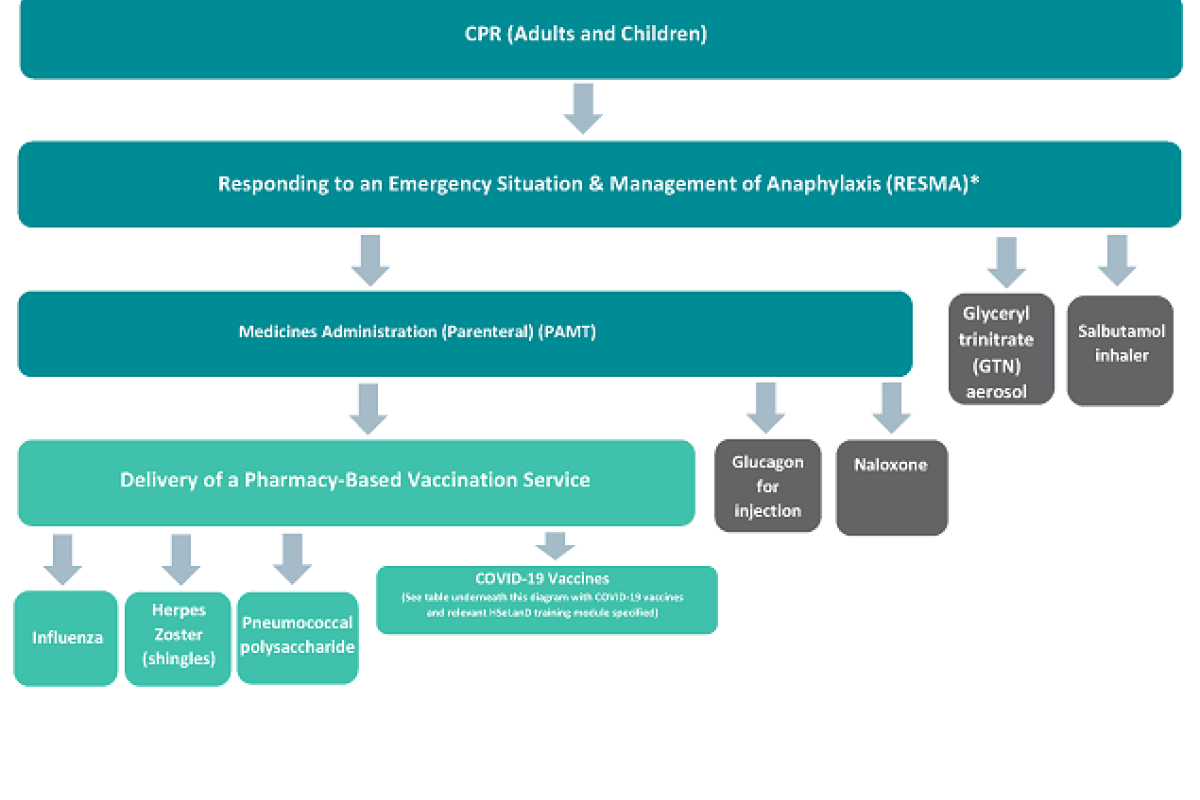
Pharmacists are deemed trained and competent in immunisation practice once they have completed the PSI approved training pathway (i.e., hold up-to-date certificates in CPR training for adults and children, Responding to an Emergency Situation and Management of Anaphylaxis (RESMA), Medicines Administration (Parenteral) (PAMT), and Delivery of a Pharmacy-Based Vaccination Service training programmes). The training programmes for the specific vaccines you wish to administer can then be completed, as set out in the diagram below and table underneath.
| COVID-19 Vaccinations used in the COVID-19 Vaccination Programme and relevant HSeLanD module to be completed as part of the required training | |
|---|---|
| COVID-19 Vaccine |
HSeLanD Module |
| Comirnaty JN.1 3mcg (for children aged 6 months to 4 years) | mRNA COVID-19 Vaccine Formulations for children aged 6 months to 4 years |
| Comirnaty JN.1 10mcg (for children aged 5-11 years) | mRNA COVID-19 Vaccine Formulations for children aged 5-11 years |
| Comirnaty JN.1 30mcg (for individuals 12 years and older) | mRNA COVID-19 Vaccine Formulations for people aged 12 years and older |
| Comirnaty KP.2 30mcg (for individuals 12 years and older) | mRNA COVID-19 Vaccine Formulations for people aged 12 years and older |
| Nuvaxovid XBB.1.5 | COVID-19 Vaccination Training Programme – Nuvaxovid XBB.1.5 |
FAQs on Seasonal Influenza vaccination
In order to supply and administer Influenza vaccines, pharmacists must first undertake or have up-to-date training in the following programmes:
- CPR for adults and children
- Responding to an Emergency Situation and Management of Anaphylaxis (RESMA)
- Medicines Administration (Parenteral) (PAMT)
- Delivery of a Pharmacy-Based Vaccination Service
- Administration of Influenza Vaccination Training Programme 2023/2024 (Available through the IIOP)
Information on the training programmes approved by PSI, as well as the providers and how to book these courses is available on the Irish Institute of Pharmacy (IIOP) website. The IIOP has also developed a new vaccination training identification tool to guide and support pharmacists in identifying their training requirements for the supply and administration of vaccinations. As well as completing the relevant training detailed above, pharmacists must also complete a self-assessment of competence.
The procedure to be followed following breakdown in the ‘cold chain’ is set out in the HSE Guidelines for maintenance of cold-chain in vaccine fridges and management of vaccine stock. In addition, the following resources may be helpful:
- Recommendations for maintenance of Vaccine Fridge within Temperature during a Planned Power-cut
- Vaccine Ordering and Storage
- 30 minute e-learning module Storing and Managing Vaccines available on HSeLanD.
No. Under Regulation 4B of the Medicinal Products (Prescription and Control of Supply) Regulations 2003 (as amended) the authority to supply and administer a vaccine in a retail pharmacy business, without a prescription, is solely vested to an appropriately trained registered pharmacist.
Information is provided by the HSE about the National Cold Chain Service.
A pharmacy may highlight the availability of a vaccination service provided by that pharmacy, to their patients and members of the public. This can include the display of factual information in relation to the pharmacy vaccination service. It must not include the brand name of a specific vaccine.
All vaccines are licensed as prescription-only medicines and the advertising of prescription-only medicines to the general public is not permitted by the Medicinal Products (Control of Advertising) Regulations 2007.
Yes. Pharmacies can provide a vaccination service at the registered pharmacy premises, outside of the normal opening hours, provided a registered pharmacist is present on site at all times. Pharmacy owners and superintendent pharmacists planning a vaccination service outside the normal opening hours of the pharmacy should ensure appropriate security is in place to safeguard their staff members and ensure patients and members of the public do not have access to the dispensary or other areas that medicines are kept.
If you wish to add a second patient consultation area to your pharmacy floor plan, you can send the PSI a copy of the updated floor plans in advance of commencing any works, outlining the changes that are to be made to the original floor plan. Pharmacies can make certain changes to their registration details using the online registration portal.
We will review these floorplans on a case-by-case basis and advise if it will be necessary to complete a Material Change Form and/or pay an associated fee. However, the need to add an additional consultation area should be considered in light of changes to legislation which allow pharmacists to deliver certain vaccination services (COVID-19 and Influenza vaccines) at locations offsite from the registered pharmacy premises (see below).
Yes. In October 2020, legislation was amended to permit appropriately trained pharmacists to supply and administer the influenza vaccine at any suitable and appropriate place (i.e., within or offsite from the retail pharmacy business premises) having regard to public convenience and the need to protect the health and safety of the public. This includes the influenza vaccine suspension for injection presented as a pre-filled syringe, and the influenza vaccine nasal spray, suspension.
Any influenza vaccination service provided offsite under this legislation must be carried out in connection with a named registered retail pharmacy business, in which the vaccinating pharmacist is employed or engaged. The PSI has published guidance to support this practice extension.
Legislation permits an appropriately trained pharmacist to supply and administer the influenza vaccine suspension for injection presented as a pre-filled syringe and the influenza vaccine nasal spray, suspension, at any suitable and appropriate place (i.e., within or offsite from the retail pharmacy business premises) having regard to public convenience and the need to protect the health and safety of the public.
Your choice of location should include a risk assessment to assure patient safety and the same high standard of care as would be provided on the pharmacy premises. Whether delivery of an influenza vaccination to a patient in an off-site location is , suitable and appropriate will, depend on a number of considerations.
We have published guidance to help you in considering what types of location are appropriate, and to support pharmacies in providing safe influenza vaccination services offsite from the pharmacy premises. There are a number of checklists included in the guidance and key points for consideration. Current PSI guidance which supports the provision of a pharmacy vaccination service in the pharmacy will still apply and must be read and adapted to the chosen setting.
Patients should have advance notice of the location and nature of the off-site vaccination site. As is the case with all vaccination services, both the patient and pharmacist should be satisfied that the given setting is appropriate for the individual patient
No. While legislation has been introduced to enable administration of COVID-19 vaccinations and Influenza vaccinations by students of health professions, including students undertaking a master’s degree in pharmacy, this applies to administration of vaccinations by vaccinators employed by the HSE. These services operate under the management and governance of the HSE.
What training do I need to complete?
The training programmes you will have to complete will depend on what services you wish to provide. You will receive a certificate of completion once you complete each training programme.
In order to supply and administer any vaccine, pharmacists must first undertake or have up-to-date training in the following programmes:
- CPR for adults and children
- Responding to an Emergency Situation and Management of Anaphylaxis (RESMA)
- Medicines Administration (Parenteral) (PAMT)
- Delivery of a Pharmacy-Based Vaccination Service
The training programmes for the specific vaccines you wish to administer can then be completed. This is illustrated in the diagram below.
Diagram illustrating pharmacist training requirements for the supply and administration of vaccines and emergency medicines

*The National Immunisation Advisory Committee (NIAC) made significant changes to the Anaphylaxis Chapter of the Immunisation Guidelines for Ireland in June 2022, which stated that “Adrenaline auto-injectors are not recommended as first line treatment by health professionals for the immediate management of anaphylaxis or suspected anaphylaxis following vaccination unless they are the only source of adrenaline available, as they may not allow IM delivery of an age appropriate dose”.
In the absence of any other National Guidelines for the immediate management of anaphylaxis in the community, PSI would consider it best practice for a pharmacist to administer Adrenaline (Epinephrine) intramuscularly from an ampoule, in all emergency circumstances (where indicated), in accordance with NIAC guidelines.
This would require the pharmacist to have valid training in:
- CPR,
- RESMA, and
- PAMT
However, if only an Adrenaline (Epinephrine) auto-injector is available, or if you are only trained and competent to administer an Adrenaline (Epinephrine) auto-injector, this should be used.
Pharmacists should use their expert knowledge, skills and professional judgement to administer Adrenaline (Epinephrine) in line with National guidance in accordance with the product readily available to them, which they are trained and competent to administer.
How can I access training and how long is it valid for?
| Training programme | Validity and where to access |
| CPR Course for adults and children | Details of providers are available on the IIOP website. Training is valid for two years (or as stated by training provider) |
| Medicines Administration (Parenteral) (PAMT) training programme | Available as a blended programme through Hibernian Healthcare. Pharmacists are asked to reflect, self-assess and to evaluate whether they need to refresh their training in this programme, in order to ensure they have the necessary skills and knowledge to safely deliver the associated medicines or vaccination service. The PSI requires that this training programme is repeated if a pharmacist wishes to administer: 1. a vaccine or emergency medicine via an injection route i.e., intramuscular or subcutaneous, which they have never previously administered; or 2. a vaccine or emergency medicine via an injection route which they have neither practised (i.e. administered to a patient) nor been trained on in the previous 12 months (or in the case of seasonal influenza vaccination, in the previous flu season) |
| Responding to an Emergency Situation and Management of Anaphylaxis (RESMA) training programme | Developed by Hibernian Healthcare and available through the IIOP. Training is valid for two years. |
| Delivery of a Pharmacy-based Vaccination Service Training Programme | Available through the IIOP. Pharmacists are asked to reflect, self-assess and to evaluate whether they need to refresh their training in this programme, in order to ensure they have the necessary skills and knowledge to safely deliver a vaccination service. The PSI requires that this training programme is repeated if a pharmacist has not vaccinated in the past 12 months (or influenza season). |
| Training for the specific vaccine(s)/emergency medicine(s) to be administered | Available either through the IIOP (influenza, pneumococcal and herpes zoster (shingles) vaccines), or in the case of the COVID-19 vaccines and Naloxone through HSeLanD. Influenza training is valid for one year. Pneumococcal and Herpes Zoster training is valid for two years. Influenza training is valid for one year. |
| Naloxone Pharmacists are required to undertake the HSeLanD online module entitled ‘Opioid Overdose Awareness and Naloxone Administration Training (Module 1)’, in addition to maintaining up-to date training in: - CPR - Responding to an Emergency Situation and Management of Anaphylaxis (RESMA) - Medicines Administration (Parenteral) (PAMT) This means that pharmacists can build on their existing skills they have developed through the delivery of vaccinations, and no longer have to undertake specific face-to-face training to supply and administer Naloxone in emergencies. Face-to-face training (Module 2) is also available through the HSE, should pharmacists wish to complete it, though this is not a mandatory requirement. |
COVID-19 Vaccinations used in the COVID-19 Vaccination Programme and relevant HSeLanD module to be completed as part of the required training
| COVID-19 Vaccine | HSeLanD Module |
| Comirnaty JN.1 3mcg (for children aged 6 months to 4 years) | mRNA COVID-19 Vaccine Formulations for children aged 6 months to 4 years |
| Comirnaty JN.1 10mcg (for children aged 5-11 years) | mRNA COVID-19 Vaccine Formulations for children aged 5-11 years |
| Comirnaty JN.1 30mcg (for individuals 12 years and older) | mRNA COVID-19 Vaccine Formulations for people aged 12 years and older |
| Comirnaty KP.2 30mcg (for individuals 12 years and older) | mRNA COVID-19 Vaccine Formulations for people aged 12 years and older |
| Nuvaxovid XBB.1.5 | COVID-19 Vaccination Training Programme – Nuvaxovid XBB.1.5 |
Emergency Medicines
| Glyceryl Trinitrate Spray | Two years - Available through IIOP |
| Salbutamol inhaler | Two years - Available through IIOP |
| Glucagon | Two years - Available through IIOP |
| Naloxone |
Two years - Available through HSELand Pharmacists are required to undertake Module 1 entitled ‘Opioid Overdose Awareness and Naloxone Administration Training’. |
In addition to completing the above training pathway, pharmacists should ensure that they are familiar with the most recent national guidance on immunisation and the management of anaphylaxis, as contained within the Immunisation Guidelines for Ireland from the National Immunisation Advisory Committee (NIAC) and Immunisation Bulletins from the National Immunisation Office. Pharmacists should be aware of updates to relevant national guidance and adapt their practices to reflect the most up to date information.
How can I complete training?
You can find out more information about training and providers on the Irish Institute of Pharmacy (IIOP) website. There you will find details on how to register and complete most of the required training programmes. Many of the training programmes are available online as e-learning programmes. There is a fee associated with the Medicines Administration (Parenteral) (PAMT) training programme which is a blended programme, involving both online and face to face components.
Training on the COVID-19 vaccines is available through HSeLanD- the Health Service Executive's online learning and development portal (NIO training). The HSE has developed this guide for community pharmacists to access HSeLanD training.
What do I need to do each year?
You should review the training requirements for the delivery of the service(s) you wish to provide each year. You should check that your training in each training programme is up-to-date. You should also complete a self-assessment and self-declaration form, which will help you to review the requirements and provide a means for you to attest to your competency to deliver the chosen service(s).
Further information and questions
If you have any questions about the training requirements, you can email cpd@psi.ie.
Useful resources
- National Immunisation Advisory Committee (NIAC) Immunisation Guidelines for Ireland
- National Immunisation Office (NIO) Immunisation Bulletins
- COVID-19 Vaccine Information for Health Professionals
- Further information on vaccination services
- Read the legislation which allows for these services.

