Inspection Findings 2016
Introduction
The PSI inspects pharmacies to assess compliance with the Pharmacy Act 2007 and other pharmacy and medicines legislation, in the interests of the health and safety of the public.

The 247 pharmacy inspections included:
- 92 new pharmacy openings
- 6 inspections relating to the internet supply of non-prescription medicines
- 149 systems inspections, including six re-inspections.
Finding from Systems Inspections
An overview of the findings from the 149 systems inspections are provided under the five areas of pharmacy practice that were reviewed by Authorised Officers during the inspections.
1. The Supply of Prescription Only Medicines, including Controlled Drugs
This area is reviewed to determine how prescription medicines are supplied to patients from pharmacies. Normally, four supplies are selected from pharmacy records and the prescriptions used to authorise these supplies are requested.
A total of 596 supplies were selected for review:
- Approximately 50% related to the supply of controlled drugs
- 5% related to ‘emergency supplies'
Of the supplies checked, the following observations were made:
General
- 92% of the prescriptions checked were in date and valid for the supply made.
- 74% of the prescriptions reviewed were written correctly, in compliance with legislation.
In cases where they were not written correctly, the main issues identified related to prescription writing requirements for schedule 2 CDs, where:
- The quantity of medicines/dosage units to be supplied was not written in both words and figures
- The name and address of the patient was not handwritten on the prescription
Emergency Supplies
The management of emergency supplies remains an area of concern, where repeated non-compliance is observed. We encourage pharmacists to read the inspector’s Advice on Emergency Supply article to ensure the pharmacy is operating in accordance with ‘emergency supply’ provisions of Regulation 8 of the Medicinal Products (Prescription and Control of Supply) Regulations 2003 (as amended).
- Only 16% of the ‘Emergency Supplies’ reviewed were carried out in accordance with legislative requirements.
The following issues were identified:
i. Schedule 2, 3 or 4 Controlled Drugs were supplied.
ii. The quantity of medicines supplied at the request of the patient exceeded the permitted quantity allowed.
iii. Where the supply was requested by a prescriber, the original prescription was not supplied to the pharmacy within 72 hours after the supply.
Click below for more information
Expand all
Collapse all

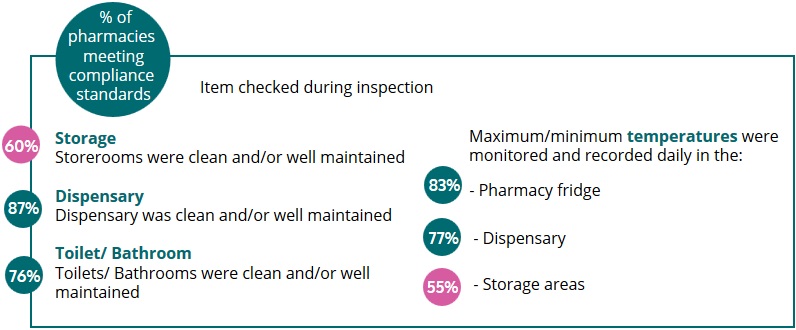
2. Pharmacy Premises and Medicines Storage
Pharmacy premises are reviewed to ensure that there is a safe and effective environment for the storage, preparation and sale and supply of medicines. Furthermore, the premises are checked to ensure that they are clean, well maintained and reflect the professional nature of a healthcare facility.
Of concern is the standard of storage areas used to store medicines and pharmacy records. All areas registered as part of a retail pharmacy business must be clean and well maintained. In addition, the maximum/minimum ambient temperatures must be monitored and recorded daily in these areas, if the area is being used for the storage of medicines and/ or nutritional supplements.
Click below for more information
Expand all
Collapse all

3. Management of Controlled Drugs (CD)
The Controlled Drugs Register is reviewed to ensure that all receipts and supplies of schedule 2 CDs are appropriately entered and accounted for. The balances of three or more schedule 2 CD preparations are checked to verify that the quantity of stock specified in the CD register corresponds with the physical quantity in the CD safe. In addition, the storage location of controlled drugs is checked to ensure that schedule 2 and 3 CDs are securely stored in a locked, controlled drugs safe.
A high level of compliance was observed in this area, but it is important to remember that the management of CDs should be regularly reviewed to ensure full compliance with the legislation.
 ;
;
Click below for more information
Expand all
Collapse all

4. Quality Management Systems
The quality management system is the system in place at the pharmacy to ensure that the pharmacy operates in a manner that is safe for patients and the public. In conducting their inspection, Authorised Officers review several documents including SOPs, Medication Error Management Records and the Duty Register.
Of particular concern is the manner in which medication errors are being managed at pharmacies. To help pharmacies improve compliance in this area we encourage you to read the Inspectors' Advice on Medication Error Management where you will also find templates for recording medication errors and near misses. This matter has also repeatedly been highlighted in the stated learnings from complaints and disciplinary proceedings.

Click below for more information
Expand all
Collapse all

5. Supply of Medicines to Patients in Nursing Homes and Residential Care Settings
Authorised Officers look at procedures and records governing the supply of medicines, the use of prescriptions, patient counselling, medication use reviews and delivery records to ensure that patients in nursing homes or residential care settings receive the same level of professional care as those patients who present in person at a pharmacy.
27% of the inspected pharmacies provided pharmaceutical services to patients in nursing homes and/or residential care settings.
Of particular concern is the management of Medicine Use Reviews. Pharmacists should make themselves available to participate in the interdisciplinary review of each patient on long term medication, and while this may be happening it is important that records of the pharmacist’s participation in these reviews are retained and available for review at the pharmacy. We encourage you to read the PSI Guidance to ensure all requirements are being met if you are providing this service at your pharmacy.

Click below for more information
Expand all
Collapse all
In light of the above, it is important that pharmacies refer to the relevant resources that the PSI has provided to assist pharmacists, pharmacy owners and pharmacy staff in meeting the standards of compliance expected under the Pharmacy Act 2007, and other medicines and pharmacy legislation. The PSI website includes guidance documents, inspectors' advice articles, videos, learning from complaints and inquiries, and newsletter updates.
The Pharmacy Assessment System is another useful tool to help assess your pharmacy's practice and facilitate a continuous cycle of ongoing compliance.The checklist for inspection is also available to help prepare for future inspections.
An overview of inspection findings are available for: